Electromagnetic water treatment Device "Termite
V. V. Bannikov, Ph.D. tech. Sciences
Director of Ecoservice Technohim
(www.etch.ru)
It is well known that the processes of scale formation and encrustations are associated with the presence in natural water, including fresh water, of large amounts of dissolved calcium and magnesium salts. These elements are undoubtedly important for humans, for the development of flora and fauna, but they cause a lot of problems in the design and operation of boiler and heat exchange equipment. We are all familiar with scale and deposits in heating devices, in pipes, in washing machines and dishwashers, lime deposits on plumbing fixtures, tiles, as well as dry hair and skin when washing with water high in calcium and magnesium.
About water hardness
Natural waters are very diverse in chemical composition. The main impurities in river waters containing 500-600 mg/l of dissolved salts are calcium, magnesium, sodium, bicarbonate, sulfate and chloride ions. Low-mineralized river waters contain mainly calcium and magnesium ions.
The salinity of groundwater depends on the conditions of occurrence of the underground horizon and varies from 100-200 mg/l to several grams per liter. The fresh waters of artesian wells are dominated by Ca 2+ and HCO 3 2- ions. These ions are present in all mineralized waters. The source of their appearance is natural deposits of limestone, gypsum and dolomites. Low-mineralized waters contain the most Ca 2+ ions. The total concentration of calcium and magnesium cations, expressed in mg-eq / l, determines the hardness of water.
The total hardness of water is also defined as the sum of carbonate (temporary) and non-carbonate (permanent) hardness. Carbonate hardness is due to the presence of calcium and magnesium bicarbonate salts and is eliminated by boiling water. When water is heated, bicarbonates decompose with the formation of unstable carbonic acid and an insoluble precipitate of calcium carbonate and magnesium hydroxide. Non-carbonate hardness is associated with the presence of calcium and magnesium in the water in the form of salts of sulfuric, hydrochloric and nitric acids. This hardness is not removed by boiling.
Hard water is unsuitable for circulating water supply systems, for feeding steam and hot water boilers, as well as for almost all types of heat exchange equipment. The deposits of hardness salts lead to a significant increase in thermal energy for heating and to an equivalent increase in fuel consumption costs. They also adversely affect the heat exchange and hydraulic characteristics, disable pumping, shut-off and control equipment, and accelerate corrosion processes.
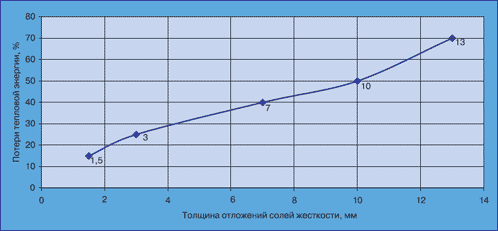
On fig. Figure 1 shows the dependence of thermal energy losses depending on the thickness of the layer of hardness deposits (according to Lifescience Products LTD, UK). A layer of 3 mm absorbs 25% of thermal energy, and if 13 mm has grown on the walls of a boiler or boiler, then 70% of heat is already lost. Deposits 10 mm thick build up in less than one year. Many are aware of the level of costs for repairs, chemical and mechanical cleaning, for the replacement of pipes and water heating equipment.
If we look at the scale problem from the point of view of excessive fuel consumption during the operation of thermal power equipment, the picture is very similar (Fig. 2).
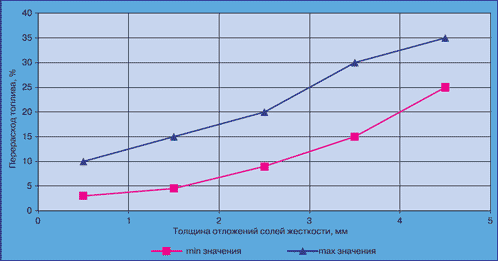
Rice. 2. Excessive consumption of fuel depending on the thickness of the scale layer on the heating surface.
From this graph it can be seen that 5 mm of scale lead to an excessive consumption of fuel up to 30%, and 10 mm - double its consumption.
Specialists of the High Voltage Research Institute are considering another important aspect of the harmful effects of scale - an increase in the temperature of the wall of a hot water (smoke or flame) pipe. For an example in fig. Figure 3 shows the dependence of the temperature of the wall of a water-heating screen pipe placed in the furnace space (temperature 1100 °C) on the thickness of the scale layer. The data are presented for various scale thermal conductivity values.
An increase in the scale layer on the heating surface of the boiler from the water side significantly increases the temperature of the wall of the hot water pipes. In turn, an increase in temperature leads to a decrease in both the tensile strength of the metal and its yield strength. In this case, fistulas are formed, and pipes burst.
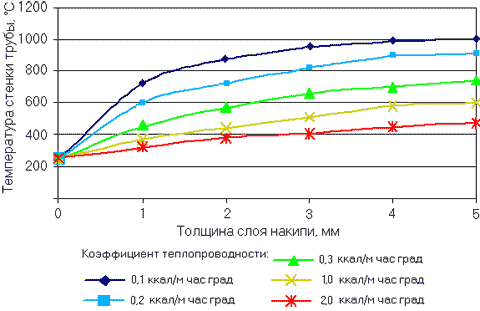
Rice. 3. Influence of scale layer thickness and its thermal conductivity on pipe wall temperature.
In accordance with GOST 2874-82 "Drinking water", water hardness should not exceed 7 mg-eq / l. However, a number of industries set more stringent requirements for process water, up to deep softening (0.01-0.05 meq/l and below). The handbook contains indicative requirements for the total hardness (mg-eq / l) of feed water for boilers of various types:
- fire tube (5-15 ati) - 0.35;
- water pipes (15-25 ati) - 0.15;
- high pressure (50-100 ati) - 0.035;
- drum (100-185 ati) - 0.005.
There are a number of ways to soften water (the process of removing Ca 2+ and Mg 2+ ions). The most common chemical method is the ion exchange of calcium and magnesium ions contained in water for sodium or potassium, which do not form precipitates of their salts when heated. In softeners of this type, a cation exchange resin works, which must be periodically regenerated with a solution of common salt. This method is not without significant drawbacks. The use of common salt for resin regeneration creates environmental problems due to the need to dispose of wash waters with a high salt content. Calcium salts are removed from drinking water below the norms required for our body, while the water is enriched with sodium, which is far from useful for drinking. The service life of ion-exchange resins is limited.
Water is also softened using membrane filters, which actually desalinate it. This method is less common due to the high cost of membranes and the limited resource of their work.
There are other softening methods: thermal, reagent, dialysis and combined. The choice of water softening method is determined by its chemical composition, the required degree of softening and technical and economic indicators.
Magnetic water treatment
In recent decades, both in Russia and abroad, magnetic water treatment has been used to combat the formation of scale and encrustations. It is widely used in steam turbine condensers, in low-pressure and low-capacity steam generators, in heating networks and hot water supply systems, and in various heat exchangers. In comparison with common methods of water softening, magnetic treatment is distinguished by simplicity, low cost, safety, environmental friendliness, and low operating costs.
The first patent for a magnetic water treatment apparatus was issued to the Belgian engineer T. Vermeiren in 1946. Back in 1936, he discovered that when water that crossed the magnetic field lines was heated, scale did not form on the heat exchange surface.
The mechanism of the effect of a magnetic field on water and the impurities contained in it has not been finally elucidated, but there are a number of hypotheses. MPEI and MGSU specialists performed a large amount of work to study the influence of a magnetic field on the processes of scale formation, developed devices for magnetic water treatment, formulated technical requirements and conditions for their use for practical purposes.
Modern views explain the mechanism of the effect of a magnetic field on water and its impurities by polarization phenomena and the deformation of salt ions. The hydration of ions during processing decreases, the ions approach each other and form a crystalline form of a salt. One of the theories is based on the influence of a magnetic field on the colloidal impurities of water, according to another, the structure of water changes. When a magnetic field is applied, crystallization centers are formed in the mass of water, as a result of which the release of insoluble hardness salts occurs not on the heat transfer surface (heating or cooling), but in the volume of water. Thus, instead of solid scale, migrating fine sludge appears in the water, which is easily removed from the surface of heat exchangers and pipelines. In magnetic processing devices, water must move perpendicular to the magnetic lines of force.
A very interesting explanation of the mechanism of magnetic water treatment is offered by V.A. Prisyazhnyuk in his work. It is known that calcium carbonate can crystallize in two modifications (calcite or aragonite), while the main salt deposited on heat exchange equipment is carbonate in the form of calcite. Magnetic treatment "forces" calcium carbonate to crystallize in the form of aragonite, which has lower adhesion (sticking) to the material of the heat exchange surface, as well as lower cohesive force (sticking) of crystals among themselves. To explain this phenomenon, the author uses the theory of magnetohydrodynamic (MHD) resonance. When liquid crosses magnetic field lines, a Lorentz force is created, which causes a structural rearrangement of carbonate (a change in the entropy of a substance) when it falls into resonance with natural vibrations of particles of a substance (molecules, ions, radicals).
At present, two types of devices for magnetic water treatment are produced in Russia - with permanent magnets and electromagnets. The residence time of water in the apparatus is determined by its speed in the range of 1-3 m/s.
The conditions for using devices for magnetic water treatment are given in the handbook:
- water heating should be carried out to a temperature not exceeding 95 ° C;
- carbonate hardness should not exceed 9 meq/l;
- the content of dissolved oxygen should be no more than 3 mg/l, and the amount of chlorides and sulfates - no more than 50 mg/l;
- the content of ferrous iron in artesian water is allowed no more than 0.3 mg / l.
To determine the antiscale effect E, %, the following expression is used:
E \u003d (m n - m m) * 100 / m n, (1)
where - m n and m m - the mass of scale formed on the heating surface during boiling under the same conditions of the same amount of water with the same initial chemical composition, respectively, untreated and treated with a magnetic field, g.
Despite all the advantages of devices for magnetic water treatment, in practice, the treatment effect often manifested itself only in the first period of operation, then the result disappeared. There was even a term - the effect of "addictive" water. Magnetized water retains its properties for less than a day. This phenomenon of loss of magnetic properties is called relaxation. Therefore, in heating networks, in addition to magnetizing the make-up water, it is necessary to treat the water circulating in the system by creating a so-called anti-relaxation circuit, with the help of which all the water circulating in the system is processed.
Electromagnetic influence
with variable frequency
At the end of the last millennium, foreign and domestic devices for treating water with electromagnetic waves in the sound frequency range appeared, which have significant advantages over devices for magnetic water treatment. They are distinguished by small dimensions, ease of installation and maintenance, environmental safety, low operating costs. The range of conditions for their use has been significantly expanded, primarily for water with high hardness, there are no high requirements for the total salt content, and the effect of water “addiction” has been eliminated. In addition, treated drinking water retains calcium and magnesium, which our body needs for the musculoskeletal, cardiovascular and nervous systems. Those. devices of this type can be used not only to protect heat exchange equipment, hot water systems, etc., but also for water treatment systems and drinking water communications. Another advantage of these devices is the destruction of previously formed deposits of hardness salts within 1-3 months.
Russia uses foreign-supplied devices Water King (Lifescience Products LTD, Great Britain), Aqua (Trebema, Sweden), as well as domestically produced devices of the Termit series (Ecoservice Technochem").
The electronic converter of hardness salts "Termite" is a wall-mounted device, available in two modifications. "Thermite" includes a microprocessor that controls the change in the characteristics of electromagnetic waves generated by the device in the range of 1 - 10 kHz. The generated signals are transmitted through wires - radiators, which are wound on the pipeline. In this case, the signals propagate to both sides of the pipeline. With the help of wires - emitters, the radiation flux is concentrated in the volume of water flowing in the pipeline.
Transmitted electromagnetic waves change the structure of hardness salts with the formation of a brittle aragonite form of calcium carbonate. In this case, a strong mixture of amorphous deposits of hardness salts is not formed, and the previously formed deposits are destroyed and carried away with the water flow.
Water during treatment does not change the salt composition, which preserves its qualities of drinking water without loss of essential chemical elements.
Devices "Termit" are produced in accordance with TU 6349-001-49960728-2000 (Hygienic conclusion No. 77.01.06.634.T.25729.08.0, Certificate of conformity No. ROSS RU.AYu64.A02379).
The device was awarded the First Degree Diplomas of the All-Russian Exhibition Center and the Ministry of Industry, Science and Technology of the Russian Federation, the Gold Medal of the All-Russian Exhibition Center and the Silver Medal of the Ministry of Industry.
Table 1
Technical characteristics of devices "Termite"
According to experts from the Swedish company Trebema, under the action of electromagnetic waves in the audio frequency range, calcium bicarbonate contained in the source water turns into insoluble calcium carbonate. In this case, carbonate is deposited not on the walls of pipes and equipment, but in the volume of water. This process is described by the following chemical equation:
Ca(HCO3)2<=>CaCO 3 + H 2 CO 3 (1)
Unstable carbonic acid dissociates electrolytically. It is also prone to the formation of carbon dioxide:
CO 2 + H 2 O<=>H2CO3<=>H + + HCO 3 - (2)
Carbonic acid destroys old lime deposits in pipes, water heaters, etc. An excess of carbonic acid shifts the equilibrium of reaction (1) to the left, i.e. leads to the re-formation of calcium bicarbonate. In practice, this means that calcium bicarbonate is again formed in the treated water after a few days (water “loses” its properties after electromagnetic exposure).
Swedish experts experimentally established:
1. A slight decrease in the pH value of water due to its acidification with carbonic acid. However, this reduction is so small that it does not increase the risk of corrosion.
2. Change in the electrical conductivity of water due to a decrease in pH.
3. Reduced surface tension and capillarity (requires less detergent).
Experimental check
At the Institute of Physical Chemistry of the Russian Academy of Sciences, an experimental verification of the efficiency of the work of hardness salt converters "Termite" (two samples) and the device "WK-3" of the company "Lifescience", Great Britain, was carried out under comparable conditions.
The tests were carried out according to the following express method. Artificially prepared solution in a volume of 2 l with a total hardness of 21.9 mg-eq / l (about 7.5 times higher than the water hardness of the Moscow River and 2.4 times higher than the permissible hardness value for systems with magnetic treatment) and pH value 7.5-7.8 were passed in the continuous circulation mode. The latter was carried out sequentially through a glass intermediate container, a steel pipe, and a fluoroplastic cylindrical cell.
Hardness salts were deposited on an aluminum disk placed at the bottom of a fluoroplastic cell.
The temperature of the circulating solution was maintained at 85+5°C. The circulation time of the solution in each experiment was 2.5 hours.
After the end of circulation, the disk was removed from the cell, washed, and dried in air at 100°C to constant weight. The amount of precipitation of hardness salts on it was determined by the difference in the weight of the disk before and after the experiment. According to expression (1), the antiscale effect was found. Two parallel experiments were carried out with each device.
The test results of electronic converters of hardness salts in aqueous solutions of various modifications and control experiments (without water treatment) are shown in Table 2.
table 2
Test results of devices of various modifications
The data given in Table 2 show that the electromagnetic effect on water with high hardness, even for a short time, can reduce the amount of hardness salt deposits formed on the walls by 24-30%. At the same time, the efficiency of all the studied devices under the same conditions (hardness level, temperature, diameter and length of the steel pipe) is approximately the same. It should be noted that in the experiments, water was not removed from the cycle, therefore, carbonic acid accumulating in the cycle, in accordance with the chemical reaction (1), led to a stationary state of the system carbonate (precipitate on the disk) - carbonate (undissolved particles in the volume of water) - bicarbonate . When water is removed from the cycle (as it usually happens in practice), the equilibrium of reaction (1) shifts to the right, i.e. antiscale effect should increase.
Subsequently, the Ecoservice Technohim enterprise, together with the Institute of Theoretical and Applied Electrodynamics of the Russian Academy of Sciences (Ryzhikov I.A. and coworkers), continued research on the effect of the operation of the Termit device on the scale formation process for flowing water systems at various temperatures.
All experiments were carried out using water from the city network (Moscow, Northern District). The water had the following composition:
- total hardness - 2.9-3.1 mg-eq/l, including carbonate - 2 mg-eq/l;
- free carbon dioxide CO 2 - 4.4 mg/l;
- general mineralization - 170-200 mg/l;
- iron - 0.14-0.18 mg / l;
- oxidizability - 7.2 mg O 2 /l;
- the ratio of calcium and magnesium - 4/1 mg/mg;
- pH value - 7.25-7.3.
In accordance with SNiP, the calculation of the saturation index of a given water with calcium carbonate (water stability) shows the value J = 0.15. This means that the water is capable of depositing calcium carbonate. SNiP allows in this case to use the magnetic method for anti-scale water treatment.
The experimental setup included a flow cell in the form of a quartz vessel with a tube, in which the test samples made of galvanized steel were placed. The temperature in the area of the samples was maintained with an accuracy of + 2 °C. Water in the cell came from the water supply network with preheating. Windings of wire-emitters of the Termit device are installed on the supply pipeline. The scale deposition time on the samples was up to 8 hours.
Experimental data showed that the greatest anti-scale effect is observed with intense boiling of water in the area where the samples are placed. When the Termit device was put into operation, the weight gain of scale on the samples was 8-12 times less than the weight gain on the same samples without water treatment.
With a decrease in water temperature (approximately 98 ° C; on the verge of boiling), the relative difference in scale gain decreased by 3-5 times. And, finally, at a water temperature of about 70 ° C, the relative difference in weight gain is negligible.
The obtained results can be explained by the significant influence of the content of carbon dioxide in the water on the process of scale formation. When water boils, the partial pressure of carbon dioxide in water decreases significantly, and the equilibrium of reaction (1) is shifted to the left. Sodium bicarbonate rapidly decomposes into carbonate ions, carbon dioxide and water:
Ca(HCO 3) 2 → CaCO 3 ↓ + H 2 O + CO 2 (3)
Intensive removal of carbon dioxide during boiling water "facilitates" the operation of the "Termite" device in terms of more intensive formation of a precipitate of insoluble calcium carbonate CaCO 3 in the volume of water, and not on the surface of the samples. With a decrease in water temperature, the removal of carbon dioxide is less intensive, and, accordingly, the antiscale effect decreases.
In parallel, the change in the structure of hardness salt deposits was also studied. In experiments on galvanized steel specimens, hardness salts were preliminarily deposited from a water flow. Next, the samples were placed in a stream of water treated with the Termit device.
The structure of the samples was studied using an atomic force microscope at a magnification of *10000. The results obtained are presented in fig. 4 and 5. It can be seen from the graphs that, without water treatment, the precipitate has a dense amorphous structure. When the Termit device is turned on (5 hours of operation), a granular structure of the sediment appears, which indicates its softening and stratification. The height of deposits also decreased by almost 2 times.
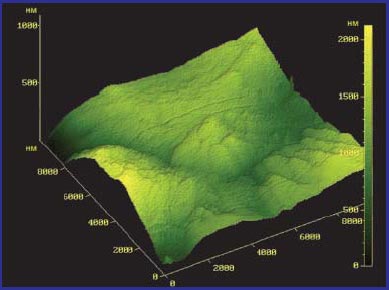
Rice. 4. Aqueous sediment of hardness salts on a steel substrate (water without treatment).
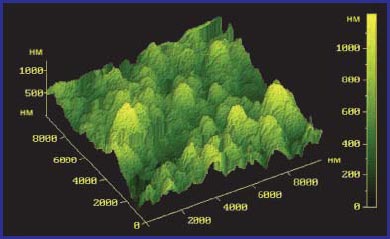
Rice. 5. Aqueous sediment of hardness salts after 5 hours of operation of the Termit device.
When selecting the type of device for electromagnetic water treatment in the range of sound frequencies (according to the diameter of the pipeline) and the optimal mode of its operation, empirical dependencies (2) and (3) should be guided.
For direct-flow water supply systems:
Q ≤ (0.005 ÷ 0.010) d² (2)
where Q - water consumption, m³ / h, d - internal diameter of the pipeline, mm.
For a system with a circulation circuit:
Qexp. / Qcirc. ≤ 0.8 (3)
where Qexp. - the amount of water taken from the system for consumption, m³ / h, Qcirc. - volume flow rate of water circulating in the system, m3/hour.
It should also be borne in mind that only carbonate hardness is subject to electromagnetic processing.
The anti-scale effect will increase(this must be taken into account when installing the device):
- by raising the temperature of the water up to the boiling point,
- at a higher content of Ca 2+ and Mg 2+ ions,
- with a decrease in the content of carbon dioxide in water,
- with an increase in the alkalinity of water,
- with a decrease in total mineralization.
- with an increase in the degree of turbulence of the water flow.
The device should be installed as close as possible to the protected equipment. If there is a centrifugal pump in the system, the electromagnetic processing device is installed after it.
Practical experience
Autonomous gas heat generators of modular type for decentralized heat supply "Geyser" manufactured by NP CJSC "Teplogaz", Vladimir.
Termit devices were installed on modular heat generators with a capacity of 240-600 kW, and Termit-M devices were installed on units with a capacity of 600-1200 kW.
During the operation of Geyser units with a capacity of 240 to 1200 kW (the area of heated premises is from 3000 to 15000 m², respectively), equipped with the Termit device, the following was noted for two years:
- periodic inspection of heat exchange surfaces (tubes) of heat generators shows that the resulting scale has a porous, easily removed structure, while the thermal conductivity practically does not decrease;
- before the use of devices, the scale had a hard, hard-to-remove structure from the surface, which led to the rapid overgrowth of the tubes;
- natural gas costs for heating are reduced by 10-15%;
- there were no shutdowns of heat generators due to the formation of scale.
Air compressor 2VM4-24/9S manufactured by the Moscow plant "Borets", Vladimir.
On the pipeline with a diameter of 50 mm for supplying artesian water in order to cool the air compressor and the aftercooler KhRK 9/8, the Termit device is installed. After the operation of the compressor for 3 months in the shop of the chemical plant, the following was noted:
- no deposits of hardness salts were observed on the surface of the water "jackets" of the compressor and the end cooler during the inspection;
- hard delaminations in the form of rusty plates were found in the cavities of the water jackets of the compressor, which were formed as a result of the destruction of the scale layer on the surface of the jackets under the influence of the Termit device;
- chemical analysis of water, both artesian and at the outlet of water from the cooled equipment, shows almost the same chemical composition (total hardness, alkalinity, chlorides, iron, sulfates, manganese).
Refrigeration unit of a meat processing plant, Penza.
The emitter wires of the Termit-M device were installed on the inlet pipeline with a diameter of 250 mm before it branched into two supply pipelines, respectively, to two plate heat exchangers MK-15. The latter function in the system of the condenser unit of the ammonia refrigeration unit.
The water from the well entering the heat exchangers had the following chemical composition:
- iron total - 0.35 mg / l,
- total hardness - 7.7 mg-eq / l,
- pH - 7.19,
- salt content - 488.7 mg/l,
- chlorides (Cl-) - 205 mg/l,
- oxidizability - 28.4 mg / l.
Water continuously circulates through plate heat exchangers MK-15.
With the specified hardness of the source water, the operation of the MK-15 heat exchangers is significantly complicated due to the very rapid overgrowth of the interplate space with hardness salts. It is required to disassemble the heat exchangers and clean them using chemical reagents.
During the operation of the "Termit-M" converter for 1-1.5 months, some accumulation of a solid precipitate of hardness salts in the interplate space of heat exchangers was noted. This circumstance is obviously related to the softening and loosening of the old precipitates of hardness salts that have formed from the surface of pipelines and heat exchangers.
After three months of testing, after opening the heat exchangers, a slight, easily removable brownish precipitate was observed on the surface of the plates. The color of the precipitate is apparently related to the incorporation of oxidized iron ions (Fe3+) and corrosion products into its structure. Difficult-to-remove, dense deposits of scale on the surface of the heat exchanger plates were not observed. This indicates that under the influence of electromagnetic radiation in the range of sound frequencies, hardness salts are converted to such a state that they either do not precipitate on the heat exchange surface, or partially precipitate in the form of a precipitate of a granular structure, which is easily removed by water flow.
Heat exchange equipment for alcohol production, Mtsensk.
Two devices of the Termit series were mounted on the cooling water supply line to plate heat exchangers to reduce the temperature of the wort from 110 to 60 °C. During operation for 1.5 years, it was possible to increase the time between cleaning of heat exchangers by 4-6 times.
The "Termit-M" device was operated for the same time on the water supply line supplying the reflux condensers and condensers of the distillation plant. The water temperature at the outlet of the installation was about 78°C. After installing the device, the time interval between cleaning the equipment increased by more than 5 times. The resulting precipitate of hardness salts has a looser structure. The dissolution of pre-existing scale was also noted.
Glass-forming machines, glass factory, Gus-Khrustalny.
Four Termit devices were installed in the recycling water supply system for cooling the technological equipment of Walter glass-forming machines. During the annual period of operation, a sharp decrease in the rate of overgrowth of heat exchange tubes with hardness salts was noted. The hard structure of scale has been eliminated, thanks to which the cooling mode of the equipment has been significantly improved.
Electrodialysis unit DVS-800M for obtaining deionized water, Podolsk.
The Termit device is installed on the water supply line to the electrodialysis apparatus in the shop of the chemical and metallurgical plant.
After installing the Termit device, the specific electrical conductivity of the filtrate decreased to 2–3 µS/cm. During 3 months of operation of the installation with the "Termit" device, the specific electrical conductivity of purified water was maintained at the level of 2.5 µS/cm, i.e. The quality of purified water in terms of the content of impurities improved by about 24%.
Thus, we can conclude that the operation of the device contributes to a more active transition of impurities from the source water to the concentrate.
Finally It can be noted that Termit devices are successfully operating at more than one and a half thousand objects. They are used to protect and clean hardness deposits from the following systems and equipment:
- plumbing communications, central heating systems;
- water heating and heating equipment - boilers, boilers, steam generators, radiators;
- equipment for purification and preparation of water, including drinking water;
- nozzles and spray devices;
- electrolyzers, electrodialysis plants;
- air conditioning systems;
- cooling systems with circulating water;
- sanitary equipment: hydromassage bathtubs, sinks, showers;
- household appliances - washing machines and dishwashers; kitchen equipment.
Literature
1. Frog B.N., Levchenko A.P. Water treatment. Moscow: MSU publishing house, 1996. 680 p.
2. Website of the High Voltage Research Institute at the Tomsk Polytechnic University. www.impulse.ru/volna, July 2004
3. Lifshits O.V. Reference book on water treatment of boiler installations. M.: Energy, 1976. 288 p.
4. Prisyazhnyuk V.A. Physico-chemical bases for the prevention of salt crystallization on heat exchange surfaces. Magazine "Plumbing, heating, air conditioning", No. 10, 2003, p. 26-30.
5. Rat D. Theory of Scale or the Practice of Magnetism, Mir Newcomer magazine, No. 1, 2002, p. 92-98.
6. Building Norms and Rules 2.04.02-84* “Water supply. External networks and structures”.
7. Building Norms and Rules 2.04.07-86* “Heat Networks. Schemes of heat networks, heat supply systems.
8. Gnedenkov S.V., Sinebryukhov S.L., Kovryanov A.N. and other Influence of coatings on the intensity of scaling processes. Institute of Chemistry, Far Eastern RAS. Electronic journal "Investigated in Russia", 2003
9. Patent of the Russian Federation No. 2174960 dated October 20, 2001 “Device for water treatment”.
Publisher: LLC IIP "AVOK-PRESS"
Specialized magazine "Energy Saving", 2005